Which Sn2 Reaction in the Following Pair Is Faster
When everything happens simultaneously it is called a concerted mechanismThis is the S N 2 mechanism. 5 xx 10 -3Wbm 2 then the induced emf between the tip of the nose and the tail of the helicopter is.
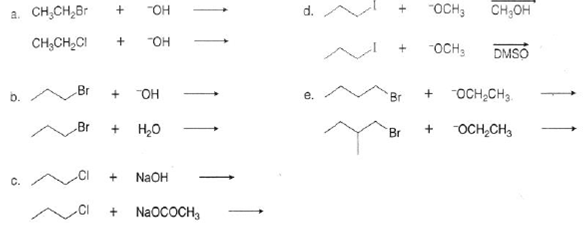
Solved Which Sn2 Reaction In Each Pair Is Faster Chegg Com
These reactions are divided in two main types.

. Step 1 of 4. Therefore I forms 3 carbocation while II forms 2 carbocation. This is the best answer based on feedback and ratings.
We review their content and use your feedback to keep the quality high. This happens because as the size increases the halide ion becomes a better leaving group. In the following pairs of compounds which would undergo SN2 reaction faster.
What will make SN2 reaction faster. Click hereto get an answer to your question Which compound in each of the following pairs will react faster in SN2 reaction with O H AMeBrI and MeIII BMe3C - ClIII and MeClIV. A helicopter rises vertically upwards with a speed of 100.
100 62 ratings for this solution. Is S N 1 faster or S N 2. SN2 with -OH.
S N 1 reaction proceeds via the formation of carbocation. Asked Mar 1 2021 in Haloalkanes and Haloarenes by RajuKumar 272k points haloalkanes. RF.
This problem has been solved. CH3CHBr will give faster SN2 reaction because when a nucleophile will approach CH2CHBr for SN2 reaction the double bond between CH2CH will hinder its approach steric effect but there is no such hindrance in case of CH3CH2Br. Which SN2 reaction in the following pair is faster.
For each of the following pairs of SN2 reactions indicate which reaction occurs faster. CH3CH2Cl Iˆ or CH3CH2Br Iˆ Students also viewed these Organic Chemistry questions For each of the following pairs of structures indicate whether the two. The first step is slow and is the rate determining.
As iodine is a better leaving group because of its large size it will be released at a faster rate in the presence of incoming nucleophile. The superposition takes place between two waves of frequency f and amplitude a. 100 23 ratings Transcribed image text.
In the S N 2 mechanism the reactivity of halides for the same alkyl group increases in the order. What is the best solvent for S N 2 reaction. Primary halide Secondary halide Tertiary halide Answered by 22nd May 2014 0346.
In the given pairs of alkyl-halide in which pair the first compound is more reactive than second compound towards S. Select the single best answer. Primary alkyl halides favor reactions.
Chemistry questions and answers. Thus the rate of reaction is faster when the leaving group is. Who are the experts.
The leaving group order of alkyl halide is as follows. Which SN2 reaction in each pair is faster. S N 2 are single step reactions and S N 1 reactions occur in two steps.
Hence out of the given pair CH3 CH 2 Br would undergo SN2 reaction faster. Hence out of the given pair CH 3 CH 2 Br would undergo S N 2 reaction faster. Ii CH 3 Cl will react faster than CH 3 3 CCl because the order of reactivity is as follows in S N 2 reaction.
The given pair of reactions is is a good leaving group than. Step 1 of 5. Polar aprotic solvent is the best solvent for S N 2 reaction as it only solvates cations and it does not alter the reactivity of nucleophiles.
For each of the given pairs indicate which substance A or B. In the following pairs which substance will react faster in a reaction. S N 2 reactions are faster as compared to S N 1.
So the reaction that takes place faster by mechanism is. One in which the nucleophilic attack and the loss of the leaving group happen at the same time and the second in which the loss of the leaving group happens before the nucleophile can attack. Greater the stability of the carbocation faster is the rate of S N 1 reaction.
Experts are tested by Chegg as specialists in their subject area. Alkanes - Reactions of Haloalkanes - Nucleophilic Substitution Reactions. Sn2 reactions are bimolecular with simultaneous bond-making and bond-breaking steps.
When the processes happen one. See the answer See the answer See the answer done loading. Primary alkyl halides prefer to undergo SN2 reactions than tertiary alkyl halides because of less steric hindrance experienced by the approaching nucleophile.
CH3CH2Br H2O or CH3CH2Br HOˆ b. Which SN2 reaction of each pair would you expect to take place more rapidly in a protic solventa 1 Or 2 b 1 Or 2 c 1 Or 2 d 1 Or 2 which SN² reaction is each pair is faster. Primary alkyl halides prefer to undergo S N 2 reactions than tertiary alkyl halides because of less steric hindrance experienced by the approaching nucleophile.
A CH3Br or CH3Cl b CH33CI or CH3l cCH3CH2Br in methanol or in dimethylformamide. The strong nucleophiles favor reactions. If the helicopter has a length of 10m and horizontal component of earths magnetic field is.
It is primary halide therefore undergoes S N 2 reaction faster. 100 8 ratings for this solution. Which compound in each of the following pairs will react faster in SN 2 reaction with OHAMeBrIandMeIIIBMe 3 CClIIIandMeClIV Hard.
Show transcribed image text Expert Answer. Classification of Haloalkanes and Haloarenes. Which SN2 reaction in.
Factors that affect the rate of reaction is as follows. The alkyl halide I is 3 while II is 2. Better leaving groups increase the rate of reactions.
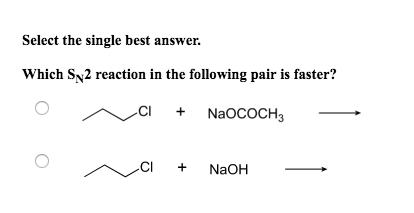
Solved Select The Single Best Answer Which Sn2 Reaction In Chegg Com
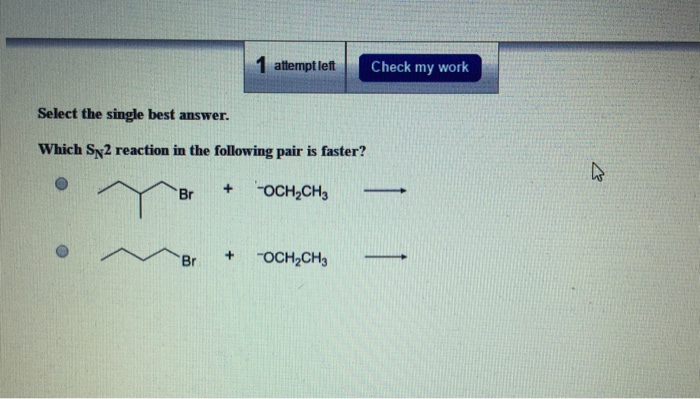
Solved Which S N2 Reaction In The Following Pair Is Faster Chegg Com
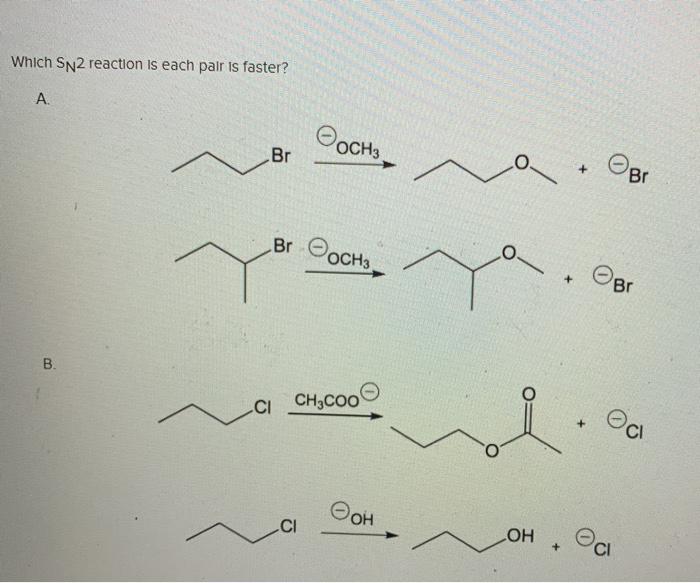
Solved Which Sn2 Reaction Is Each Pair Is Faster A Oosno Chegg Com
Comments
Post a Comment